When it does not form quartz, silicon, which has 4 available bonds, binds with 4 oxygen atoms, each of which allows one bond of the two it has available to be free. The 4 oxygen atoms orient themselves around the silicon atom so that they are as far apart from each other as possible, ending up arranged according to the direction of the 4 vertices of a tetrahedron, a solid with 4 faces in the shape of an equilateral triangle. In practice, the 4 bonds thus oriented in space resemble a tripod with 4 identical arms. In jargon, however, geologists call it a “silica tetrahedron.” When one of these tetrahedra bonds with iron and/or magnesium it forms minerals known as Olivines. Adding a molecule of SiO2 to an Olivine results in a Pyroxene, which is formed by chains of tetrahedra with metals or alkalis in the spaces. Adding SiO2 and water again we get Amphiboles, which instead have a double chain of tetrahedra.
Translated with DeepL.com (free version)
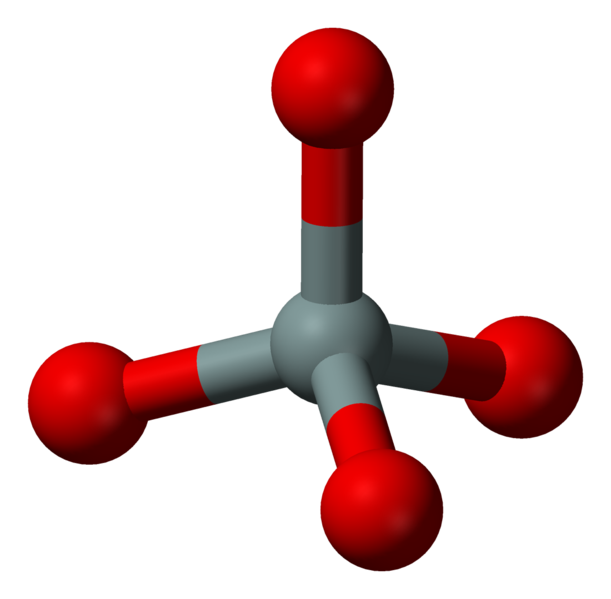
![]() The most simple type of silicates are the “nesosilicates”, those with isolated tetrahedrons SiO44- (left). Each tetrahedron can bond iron or magnesium, both bivalent, forming the minerals known as Fayalite Fe2SiO4 and Forsterite Mg2SiO4, respectively. As the Fe and Mg percentage in the crystal lattices is variable, the minerals are referred to as Olivine “solid solution”, also know as Peridot. The mineralogical formula is (Fe,Mg)2SiO4, with variable content of the two minerals.
The most simple type of silicates are the “nesosilicates”, those with isolated tetrahedrons SiO44- (left). Each tetrahedron can bond iron or magnesium, both bivalent, forming the minerals known as Fayalite Fe2SiO4 and Forsterite Mg2SiO4, respectively. As the Fe and Mg percentage in the crystal lattices is variable, the minerals are referred to as Olivine “solid solution”, also know as Peridot. The mineralogical formula is (Fe,Mg)2SiO4, with variable content of the two minerals.
By adding one silica molecule to Olivines we obtain the silicates known as Pyroxenes, where tetrahedrons are spatially organized in chains, so that they’re called “inosilicates” (right). Bonded cations may be various: (Fe,Mg,Ca,Na,Al)Si2O6. For example, by adding silica SiO2 to the magnesian Olivine “Forsterite” we have 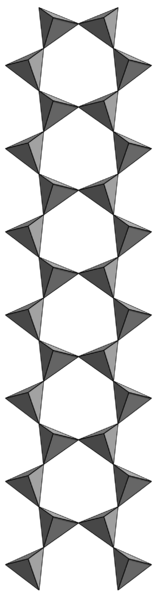 Mg2SiO4 + SiO2 = Mg2Si2O6, which is a Pyroxen known as Enstatite. Doing the same thing to the ferrous Olivine we would obtain Ferrosilite. In nature, we can find some calcium atoms in place of one iron or magnesium atoms. This would lead to Hedembergite or the more common Diopside. CaMgSi2O6.
Mg2SiO4 + SiO2 = Mg2Si2O6, which is a Pyroxen known as Enstatite. Doing the same thing to the ferrous Olivine we would obtain Ferrosilite. In nature, we can find some calcium atoms in place of one iron or magnesium atoms. This would lead to Hedembergite or the more common Diopside. CaMgSi2O6.
Going on like that we could ideally obtain the double-chain inosilicates called “Amphiboles” (left), whose more complex formula contains the hydroxylic ion Si8O22(OH)27- obtained by adding silica and water to a Pyroxene in high temperature and pressure environments typical of metamorphism.

















